The Parel Duchenne and Becker: a national biobank
Background
Trial readiness and reduction of patient burden is essential in view of the number of compounds currently in development for Duchenne and Becker (DBMD) patients. This can be facilitated by collecting natural history data from clinical care. For this, two intertwined projects have been initiated by the Duchenne Centre Netherlands (DCN). DCN was established in 2016 as collaboration between the Leiden University Medical Center (LUMC), the Radboudumc and Kempenhaeghe-MUMC +, the Duchenne Parent Project (DPP) patient organization and ‘Spierziekte Nederland’ (SN) patient association.
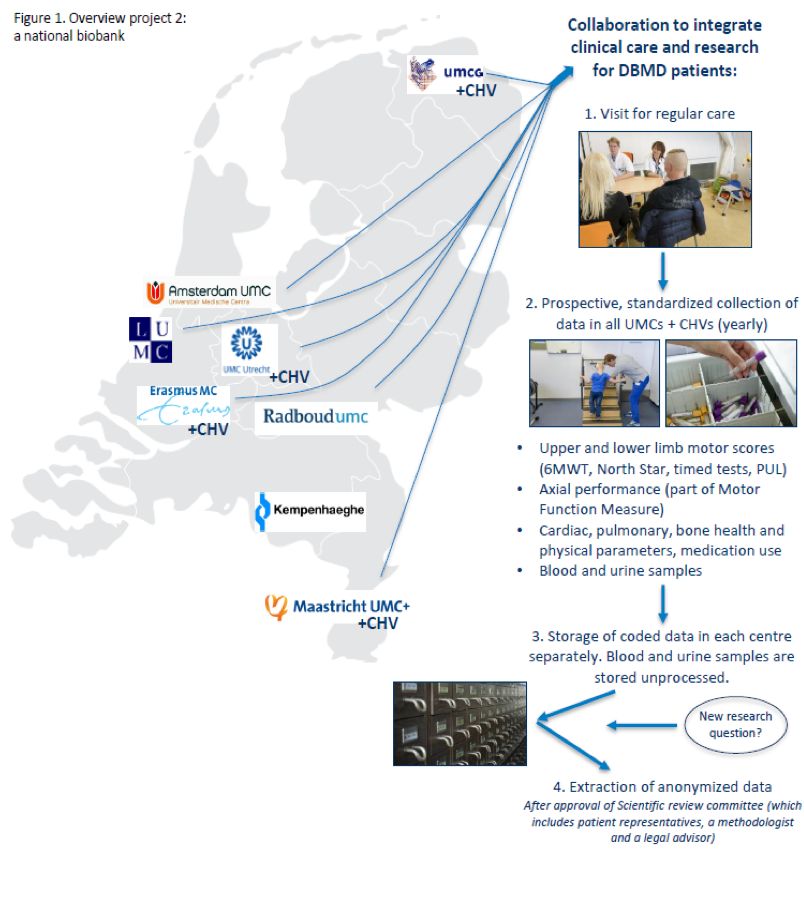
The second project is a National Biobank which was set-up to integrate clinical care and scientific research. The Biobank is a collaboration between DCN, the other 5 Dutch university medical centres (UMCs) and the 4 centers for home ventilation (CHV), and is facilitated by the Parelsnoer Institute (PSI), an initiative of the Netherlands Federation of University Medical Centres.
Methods
In this Biobank, standardized data from clinical care is collected prospectively and anonymously, including blood and urine samples. The collected dataset is consensus derived (all UMCs providing care for DBMD were involved in decision making) and based on the national care guidelines for DMD. It includes yearly assessment of motor function via the 6MWT, the North Start Ambulatory Assessment and Timed Function Tests in ambulant patients, and the Performance of the Upper limb and axial performance taken from the Motor Function Measure for all patients. To this end, all UMC pediatric physiotherapists involved in care for DBMD have been trained according to international standards for clinical trials. Yearly evaluation of cardiac and pulmonary function and bone health is included as well as physical parameters and medication use. The databases for each center are built using the web based Castor EDC data management system. Variables are similar to those used in the first project (DDD). Both projects implement Snomed and Human Phenotype Ontology nomenclature to enhance information exchange under Findable Accessible Interoperable and Reusable (FAIR) principles. Data are coded in each center. Anonymized data can be extracted after a brief study protocol has received approval from the Scientific Review Committee including patient representatives, a methodologist and a legal advisor.
Relevance
This unique project, embedded in clinical care, provides the opportunity to collect longitudinal clinical data and blood and urine samples for biomarker studies from a large cohort of Dutch DBMD patients from all ages, without predefined study end-points. This allows for research questions to be defined afterwards and data to be used natural history controls in future studies.
Financiering: Spieren voor Spieren