Dutch Dystrophinopathy Database
A national register for Duchenne and Becker muscular dystrophy patients in the Netherlands
Background
Trial readiness and reduction of patient burden is essential in view of the number of compounds currently in development for Duchenne and Becker (DBMD) patients. This can be facilitated by collecting natural history data from clinical care. For this, two intertwined projects have been initiated by the Duchenne Centre Netherlands (DCN). DCN was established in 2016 as collaboration between the Leiden University Medical Center (LUMC), the Radboudumc and Kempenhaeghe-MUMC +, the Duchenne Parent Project (DPP) patient organization and ‘Spierziekte Nederland’ (SN) patient association.
The first project is the registration database (Dutch Dystrophinopathy Database, DDD) that was originally founded in 2008 by the LUMC. Due to the collaboration of DCN and the new privacy regulations, the database has been renewed.
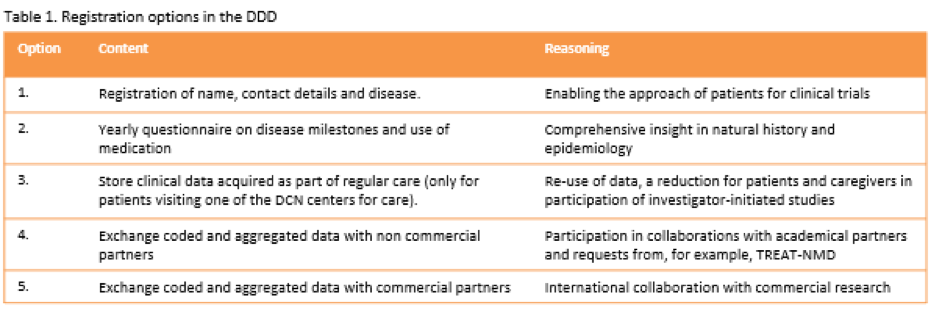
Methods
The renewed database has five different registration options to provide low-threshold registration for patients. See table 1. Only the first registration option is obligatory. With this option, patients register by name and contact details so they can be approached for new research. The other registration options allow us to get more insight in the natural history of DBMD and to collaborate with international partners.
Relevance
We will have a complete overview of all dystrophinopathy patients in the Netherlands. In this way, we facilitate the approach of eligible patients for research. In addition, part of the research burden on patients can be reduced because of re-use of data. The renewal of the database also gives the opportunity to collaborate more efficiently in an international research context.
Financiering: Spieren voor Spieren