Amyotrophic Lateral Sclerosis (ALS)-on-a-chip
Amyotrophic Lateral Sclerosis (ALS)-on-a-chip: Towards patient-specific models for personalized therapy development
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder that affects upper and lower motor neurons in 1:400 people. It results in progressive atrophy of skeletal muscles and ultimately results in death within 3-5 years after diagnosis, due to respiratory failure. More effective therapeutic strategies for ALS are urgently needed. This will require insight into the pathogenesis of ALS, as well as new disease models.
The hallmark of ALS is degeneration of motor neurons and loss of muscle innervation. A major obstacle in studying ALS has not only been the lack of human motor neurons but also the limited opportunities for studying the effects of genetic mutations or gene expression changes in a representative neuromuscular model in vitro. Recent studies confirm the feasibility of establishing such models using iPSC-generated motor neurons and muscle components. Such models, however, are typically done on a hard surface (2-dimensional), or lack in throughput, which is crucial for the testing of large numbers of potential therapeutic compounds.
In this project, we intend to develop 3-dimensional (3D) in vitro neuromuscular unit model. Using stem cell technology, the models can be generated in a patient-specific manner. The model will comprise muscle cells and motor neurons and will be further complemented by inclusion of microglia. Such models will be established in the OrganoPlate, a microfluidic platform which allows 3D cell culture, enabling a medium to high throughput with 40-96 individual tissue culture chips per device. The different cell types mentioned earlier can be embedded in extracellular matrix (ECM) and added to specific lanes within each microfluidic chip, allowing specific cell types to be interrogated with high specificity.
In order to provoke a breakthrough in understanding and treatment of ALS, we thus aim to develop, investigate and screen disease models that capture the complexity of ALS on a patient-specific basis. Such models and assays will be used for determining disease mechanisms on a ‘per patient’ basis, both for mechanistic insight and for the discovery of effective therapeutic compounds in a ‘personalized medicine’ manner.
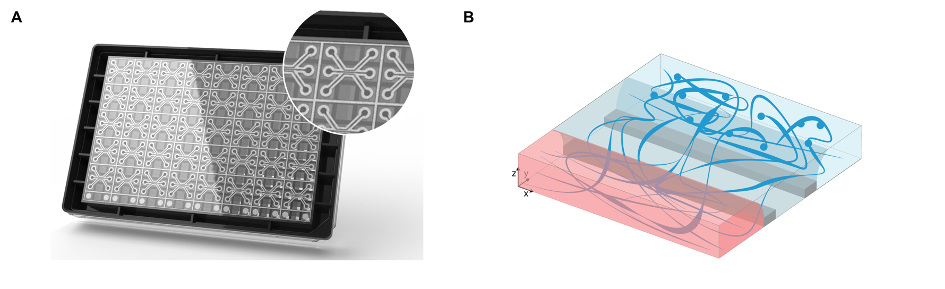
Figure – Segregated axonal outgrowth in 3D. (A) The OrganoPlate is a microfluidic device which allows 3D cell culture in a high-throughput manner. (B) Generation of a segregated axonal outgrowth model, in which neurites could be separated from the cell bodies. This separation allows researchers to study motor axon biology more specifically. In short, motor neuron progenitors (‘dark blue) are cultured in the top lane of a tissue culture chip, embedded in an extracellular matrix (ECM, ‘light blue’). ECM only is added to the middle lane of the same chip, while culture medium (‘red’) is added to the bottom lane. Over time, the motor neurons start extending their neurites into the middle lane, as such segregating motor axons from the cell bodies. It is expected that the model will be used as the starting point for a neuromuscular junction model, which constitutes motor axons innervating skeletal muscle cells.
Team members
Jeroen Pasterkamp
Paul Vulto
Nienke Wevers
Xandor Spijkers
MIMETAS BV
Financiering: Stichting ALS Nederland